What Is Gold Vermeil?
What Is Gold Vermeil and Why You Should Care!
This month's focus is all things gold vermeil. What is it? How do you pronounce it? How is it made? All this and more will be answered and we've even included a buyer's guide to gold vermeil to help you navigate the tricky world of online shopping. Gold vermeil is simply a sterling silver item that has been plated with a specific thickness and purity of gold. It is a less expensive way to have an item that appears to be gold. It is pronounced vur-may and can be a very attractive addition to your jewelry collection.
A Brief History of Plating and Gilt Objects:
Covering an object with gold is not a modern activity. Archeological findings and ancient sources tell us that it was quite common in antiquity. According to Herodotus, the ancient Egyptians used to add gold to the surface of precious objects, such as shrines [Herodotus par 22].

To achieve this, gold sheet was mechanically applied or chemistry was used to dissolve the gold into a solution which could then be brushed onto the surface of a less precious material. It wasn't until the advent of electroplating, that a durable and perhaps more importantly, a thin coat of gold, could be reliably affixed to the surface of metal. "Deposits of metal on metal surfaces by electroplating can be as thin as one-millionth of an inch..." [Choate 186]. The thinness of electroplating means that you can use much less gold to uniformly cover the item in question which reduces the cost. Electroplating was first achieved by the Italian chemist Luigi Brugnatelli in 1805 when he plated two silver medals with gold.

He used as his source of electricity, a voltaic pile invented by Alessandro Volta. This device was essentially a battery created by stacking discs of zinc on top of discs of copper with pieces of cloth saturated in salt water sandwiched between them [Engineering and Technology Wiki]. This created one to three volts of usable electricity that could be directed through wires.
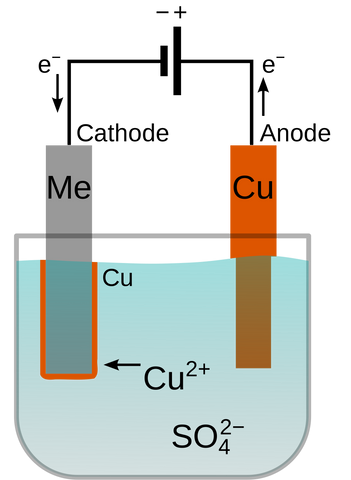
How is the Gold Plated onto the Surface of the Silver?
By the 1840s, several technical innovations had improved the process enough to make it effective and cheap. It was patented by the company Elkington & Mason who used it to electroplate silver onto cutlery. Modern electroplating is extremely effective and is accomplished in much the same way as it was 213 years ago. In the case of gold vermeil jewelry, the items must be beautifully smooth and finished. Any imperfections will be highlighted by the plating as it will look frosty instead of shiny. Once the jewelry is properly finished, it must be thoroughly cleaned and is then ready to be immersed in the plating bath (electrolyte). A circuit is created in which the silver jewelry becomes a negatively charged electrode (more specifically a cathode). Positively charged gold ions in the plating bath are attracted to the negatively charged silver and will begin to deposit on the surface of the metal. The longer you leave the item in the bath the more gold will be deposited on it.
The Legal Side of Things:
If a jeweler markets their items as gold vermeil, per FTC regulations it must be gold plating of a specified quality plated over sterling silver (925). Sterling is an alloy made up of 92.5% pure/fine silver with a permitted tolerance of .004% [JVC Guide]. The remaining 7.5% is copper [Choate 12].
The plating must be 10 karat gold or purer since anything below 10 karats cannot be marketed as gold at all. The karats denote the purity of the gold in question where 10 karat equals 41.67% gold and 24 karat equals 100% pure gold. At Charmworks we always plate in 22 karat (91.6% pure) gold. We really like the color and it is about as pure as you can get while remaining durable as pure gold is quite soft.
If your base metal is sterling silver and your plating metal is 10k or purer gold, you have now met two of three qualifications for marketing something as gold vermeil. The last qualifier is the thickness of the plating. Plating is measured in microns or millionths of an inch. For vermeil, one must plate at least 2.5 microns or 100 millionths of an inch of gold onto the surface. By comparison flash plating can be less than .175 microns or less than 7 millionths of an inch [JVC Guide]. That’s pretty thin and will probably wear off fairly quickly!
3 Legal Qualifications of Gold Vermeil:
- Base metal of 925 sterling silver
- Plated with 10 karat gold or purer
- The plating must be 100 mils/2.5 microns or thicker
The Buyer's Guide to Gold Vermeil:
There are some questions you need to be asking when you're out shopping for gold vermeil Jewelry.
- What Base Metal Are you Getting?: When you're shopping for Gold Vermeil jewelry online, try to look for sellers that explain what metal they are plating over. It's easy to plate over non-precious base metals with gold and charge precious metal prices. If it is sterling, it should be stamped with a 925 somewhere on the surface.
- How Thick Is That Plating?: They should also be plating at a minimum of 100 mils of quality gold. If they do not list this, it might be a good idea to ask them or move on. As stated above, anything thinner is called flash plating and is not vermeil!
- Why Is The Price So Cheap?: Watch out for low pricing. If the seller has very low pricing, I would maintain a healthy skepticism about the material they are trying to sell you. It could be that they have plated over pot metal or zinc with low quality gold or even a gold looking alloy. Do not let yourself be fooled! If you really like the charm, at the very least you should reach out to the jeweler and ask what materials they are using. You may find out that you get what you pay for!
You might be asking yourself, "Why buy vermeil in the first place?!?:
This is purely subjective, but there are many reasons to purchase vermeil instead of solid gold. First off, there is the price! A solid gold piece of jewelry can be more than sixteen times as expensive as a piece of vermeil jewelry. Another reason might be the color. Frequently, items cast in gold cannot be made of very pure gold as it is too soft to stand up to wear and tear. More often you'll find alloys that are 58% gold (14 Karat) with the remaining metal being silver. This creates a paler color while pure gold can be very warm and rich. We use 22 karat gold in our plating and we absolutely love the color it gives us!
References:
Choate, Sharr. Creating Casting, Crown Publishers, Inc., New York, 1966
Choate, Sharr. Creative Gold- And Silversmithing, Crown Publishers, Inc., New York, 1970
Engineering and Technology History Wiki, https://ethw.org/Electroplating, Accessed September 26th 2018
Herodotus, An Account of Egypt. The Gutenberg Project, https://www.gutenberg.org/files/2131/2131-h/2131-h.htm, Accessed September 25th 2018.
Jewelers Vigilance Committee, The Essential Guide to the U.S. Trade in Gold and Silver Jewelry, Jewelers Vigilance Committee, New York
1 comment
Thank you for that very interesting explanation!
